Korea Pharma
Laboratory overview
Excellent talent staffs and differentiated research and development(R&D),
KOREA PHARMA Central Research Institute.
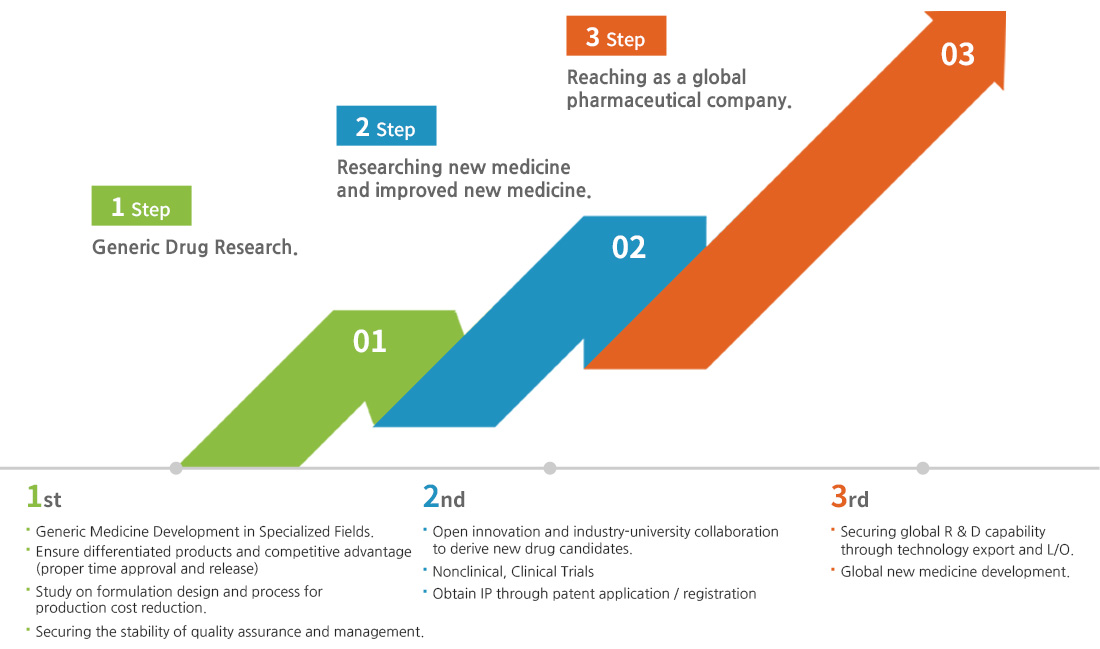
Established in 1999 with the founding philosophy of "a corporation for the health and happiness of mankind," the Central Research Institute is committed to the development of new drugs, including excellent pharmaceuticals production and improved new medicine, through securing and cultivating industrial-educational cooperation and professional manpower.
In addition, we are continuing research on generic medicine and differentiated new medicine (slow-release medicine, complex medicine), synthetic new medicine, and natural new medicine.
At the same time, we are spurring R & D for specialized technology research and intellectual property rights.
Research field Portfolio
-

The development of generic
& new medicine.- Tablet, capsule, Cream, Liquid for external use
- Research on various dosage form and commercialization
Development of first generic medicine in specialized areas and development of new medicine
(slow-release medicine, complex medicine).
-

New Medicine Research
(Synthetic medicine, Natural medicine)- New medicine development, non-clinical, clinical trial through industry-university cooperation and open innovation.
- manufacture of test medicine for clinical trials
(Securing stability) - Set new medicine specifications
(Including raw materials, product standards and test method setting and permission data setting)
-

Analysis study
- Identification of Pharmaceutical Equivalence
(Instrumental analysis and physicochemical analysis) - Development of analytical methods for new product and new medicine development
- Product standards and test methods
- Identification of Pharmaceutical Equivalence
-

Researching Infrastructure Technologies
- Study on emission control using "DDS (Drug Delivery System)".
- Studies on solubilization of poorly soluble materials.
(Emulsion, Solid dispersion) - Special formulations.
(Oral Disintegration, Mini-Tablet) - Formulation modification technology.
(tableting of soft capsules and liquids) - Shielding the taste of liquid and powder.
- Long acting Release (Depot)
Becoming a new product development center and global new drug development research center
-
- 2018
- Launched generic medicine in capsules form of the first slow-release mini-tablets in Korea.
-
- 2017
- Evaluation of efficacy and stability for the development of Alzheimer's dementia medicine for new medicine candidates material.
